Canine degenerative myelopathy (DM) is a progressive disease of the spinal cord similar to amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) in humans.1 The disease, as a result of degeneration of the spinal cord, results in the eventual loss of use of the hind legs.
Age of onset is generally after 7 years and most often seen in German Shepherds, Boxers, and Pembroke Welsh Corgis, though the mutation has also been found in a number of other canine breeds.
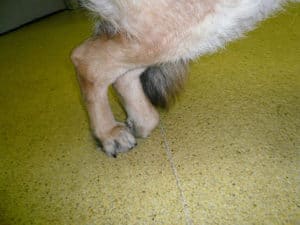
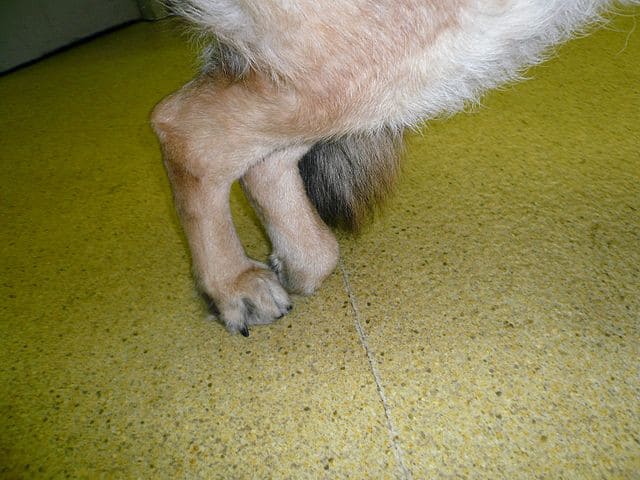
A dog standing with its hind legs together due to degenerative myelopathy.
Causes of degenerative myelopathy
Though the cause of degenerative myelopathy is currently unknown, research strongly indicates that mutations in the super-oxide dismutase 1 (SOD1) gene are associated with developing DM1. Normally, SOD1 functions as a protector of sorts, destroying harmful free superoxide radicals in cells.
Mutations in the SOD1 gene can allow free radicals to accumulate and cause damage to cells due to the loss of functioning SOD1 enzyme. Current research also suggests that mutant SOD1 causes toxic build up, resulting in cell damage and death. In humans, the SOD1 gene has been implicated in the cause of ALS.2, 3
Normally, the spinal cord acts as a communication line between the brain and the limbs. In DM-affected dogs, this line is essentially cut and the loss of hind limb use results. These mutations in SOD1 are believed to cause the loss of myelin sheaths (which acts as insulation for the spinal cord) and eventually the loss of neurons in the spinal cord, resulting in the ‘loss of communication’ to the hind limbs.4
Symptoms of degenerative myelopathy
Symptoms of degenerative myelopathy typically involves the progressive loss of muscle strength and motor coordination in the hind legs, resulting in a wobbly gait and, eventually, the dragging of hind legs when the dog walks. Other symptoms of DM include loss of balance and incontinence. Further progression leads to the complete loss of function and paralysis in the hind legs.4
Diagnosis
Previously, degenerative myelopathy was diagnosed through the elimination of other possible causes, such as spinal cord tumors, disc protrusions, or herniated discs, all of which mimic DM symptoms. More recently, DNA tests can be conducted to determine the presence of a mutated SOD1 gene.4
The chances of a particular dog developing DM as a result of genetics depends on the genetic make-up of its parents. Animals typically have two versions, or alleles, of a gene, one from each parent. Normal alleles are usually denoted as ‘N’ while mutated alleles are denoted as ‘A,’ for abnormal.
For example, a particular dog might be N/A for a particular gene, indicating that it has one normal copy and one abnormal copy of a gene. N/N represents normal, while N/A and A/A respresent carrier and at risk, respectively. A ‘normal’ animal does not carry the SOD1 mutation and is highly unlikely to develop DM.
A carrier has one mutated copy of the gene, but is unlikely to develop DM. They will, however, be able to pass on their mutated gene to offspring. An animal that is ‘at risk’ carries two copies of the mutant SOD1 gene and is at risk for developing DM.
The possibility of inheriting a mutant copy of a gene can be calculated by the parent’s genetics as follows:
- Both parents are N/N: All offspring will be normal.
- N/N x N/A: 50% of the offspring will be normal and the other 50% will be carriers.
- N/N x A/A: All of the offspring will be carriers.
- N/A x N/A: 25% of the offspring will be normal, 50% will be carriers, and 25% will be at risk.
- N/A x A/A: 50% will be carriers and 50% will be at risk.
- A/A x A/A: All of the offspring will be at risk.
Is Degenerative Myelopathy Treatable?
Currently, there are no known treatment methods to slow or stop the progression of degenerative myelopathy. The quality of life for the canine can be improved, however, through veterinary care, physical rehabilitation, and increased mobility through the aid of dog lift harnesses or wheelchairs for dogs.
DNA Statistics from the University of Missouri and Orthopedic Foundation for Animals
Below are statistics of Degenerative Myelopathy DNA testing conducted by the University of Missouri and the Orthopedic Foundation for Animals. Note that these are only test results and not indicative of a particular breed’s chances of developing DM.
Reveal Breed Statistics
| Breed | Normal | Carrier | At Risk | Total Tested |
|---|---|---|---|---|
| Akita | 13 | 2 | 2 | 17 |
| American Eskimo Dog | 11 | 7 | 4 | 22 |
| American Pit Bull Terrier | 13 | 5 | 5 | 23 |
| Australian Shepherd | 19 | 9 | 14 | 42 |
| Basenji | 28 | 0 | 0 | 28 |
| Belgian Malinois | 19 | 0 | 1 | 20 |
| Bernese Mountain Dog | 1043 | 1248 | 278 | 2569 |
| Bloodhound | 124 | 112 | 15 | 251 |
| Border Collie | 16 | 4 | 3 | 23 |
| Borzoi | 619 | 227 | 24 | 870 |
| Boxer | 473 | 969 | 1195 | 2637 |
| Canaan | 55 | 42 | 13 | 110 |
| Cardigan Welsh Corgi | 265 | 197 | 72 | 534 |
| Cavalier King Charles Spaniel | 8 | 16 | 11 | 35 |
| Chesapeake Bay Retriever | 931 | 962 | 190 | 2083 |
| Chinese Crested | 26 | 2 | 0 | 28 |
| Collie | 65 | 34 | 14 | 113 |
| Coton De Tulear | 37 | 8 | 0 | 45 |
| Czechoslovakian Wolfdog | 22 | 17 | 2 | 41 |
| Dalmatian | 63 | 0 | 2 | 65 |
| French Bulldog | 28 | 11 | 4 | 43 |
| German Shepherd Dog | 2044 | 1262 | 649 | 3955 |
| Golden Retriever | 142 | 4 | 3 | 149 |
| Great Dane | 21 | 0 | 0 | 21 |
| Great Pyrenees | 27 | 7 | 2 | 36 |
| Hovawart | 32 | 23 | 16 | 71 |
| Hybrid | 100 | 28 | 104 | 232 |
| Jack Russell Terrier | 14 | 3 | 4 | 21 |
| Kerry Blue Terrier | 179 | 153 | 43 | 375 |
| Komondor | 9 | 9 | 2 | 20 |
| Labrador Retriever | 146 | 9 | 12 | 167 |
| Mastiff | 50 | 20 | 1 | 71 |
| Pembroke Welsh Corgi | 236 | 787 | 1121 | 2144 |
| Poodle | 411 | 61 | 2 | 474 |
| Pug | 114 | 53 | 17 | 184 |
| Puli | 84 | 29 | 6 | 119 |
| Pumi | 22 | 4 | 0 | 26 |
| Rhodesian Ridgeback | 1463 | 1024 | 163 | 2650 |
| Rottweiler | 30 | 2 | 1 | 33 |
| Shiloh Shepherd | 176 | 55 | 3 | 234 |
| Soft Coated Wheaten Terrier | 9 | 10 | 7 | 26 |
| St. Bernard | 18 | 12 | 2 | 32 |
| Tamaskan | 41 | 19 | 2 | 62 |
| Weimaraner | 32 | 0 | 1 | 33 |
| Welsh Terrier | 17 | 8 | 4 | 29 |
| White Shepherd | 21 | 9 | 4 | 34 |
References
- Morgan BR, Coates JR, Johnson GC, Shelton GD, Katz ML. “Characterization of thoracic motor and sensory neurons and spinal nerve roots in canine degenerative myelopathy, a potential disease model of amyotrophic lateral sclerosis”. J Neurosci Res. 2014 Apr;92(4):531-41. doi: 10.1002/jnr.23332. Epub 2013 Dec 21.
- Milani P, Gagliardi S, Cereda C, et al. “SOD1 Transcriptional and Posttranscriptional Regulation and Its Potential Implications in ALS”. Neurology Research International. 2011; 2011: 458427.
- Awano T, Johnson GS, Wade CM, Lindblad-Toh K, Coates JR, et al. “Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis”. Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2794-9. doi: 10.1073/pnas.0812297106. Epub 2009 Feb 2.
- “Degenerative Myelopathy – Disease Basic“. Canine Genetic Diseases Network. http://www.caninegeneticdiseases.net/DM/basicDM.htm. Retrieved 2014-03-14.
- “DM Test Result Statistics“. Orthopedic Foundation for Animals. http://www.offa.org/stats_dna.html?dnatest=DM. Retrieved 2014-03-14.






Leave A Comment